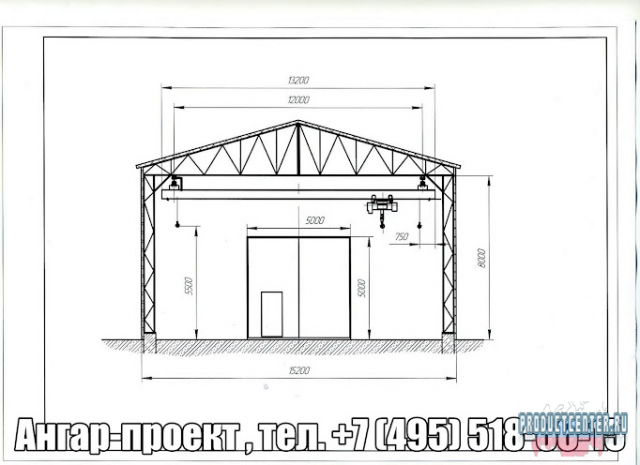
Jul 4, 2013 - Drawings KMD hangar (garage) of a metal frame in 5h15m axes, a total weight of 6,8t. Attached wiring diagram and general data, detailed. Daily 0.8331. -i-otdelochnye-materialy/7323-stroika-metallicheskogo-angara.html 0.5858.
The aim of this study was to purify and characterize a keratinase produced by a new isolated Bacillus subtilis KD-N2 strain. The keratinase produced by the isolate was purified using ammonium sulphate precipitation, Sephadex G-75 and DEAE (diethylaminoethyl)-Sepharose chromatographic techniques. The purified enzyme was shown to have a molecular mass of 30.5 kDa, as determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis. The optimum pH at 50 °C was 8.5 and the optimum temperature at pH 8.5 was 55 °C. The keratinase was partially inactivated by some metal ions, organic solvents and serine protease inhibitor phenylmethanesulfonyl fluoride (PMSF).
Sodium dodecyl sulfate (SDS) and ethylene diamine tetraacetic acid (EDTA) had positive effect on the keratinase activity. Reducing agents including dithiothreitol (DTT), mercaptoethanol, L-cysteine, sodium sulphite, as well as chemicals of SDS, ammonium sulfamate and dimethylsulfoxide (DMSO) stimulated the enzyme activity upon a feather meal substrate. Besides feather keratin, the enzyme is active upon the soluble proteins ovalbumin, bovine serum albumin (BSA), casein and insoluble ones as sheep wool and human hair. Calf hair, silk and collagen could not be hydrolyzed by the keratinase. Bacteria and growth conditions Strain KD-N2 was screened from a local poultry plant and kept in our laboratory (Cai et al., ). The medium (pH 7.2) used for keratinase production contained the following constituents (g/L): NaCl 0.5, KH 2PO 4 0.7, K 2HPO 4 1.4, MgSO 4 0.1 and feathers 10.
Cultivation was performed using 500 ml Erlenmeyer flasks containing 100 ml medium for 24 h at 28 °C with constant shaking at 200 r/min. As inocula, 5% (v/v) bacteria grew in Luria-Bertani broth [peptone 1% (w/v), yeast extract 0.3% (w/v) and NaCl 0.5% (w/v), pH 7.2] for 20 h. Culture supernatants obtained after centrifugation at 8000× g for 20 min were used for further study. Assay of keratinase activity Keratin azure (Sigma-Aldrich, USA) was used as the substrate. It was first frozen at −20 °C and then ground into a fine powder.

The 5 mg keratin azure powder was suspended in 1 ml 50 mmol/L Tris-HCl buffer (pH 8.0). The reaction mixture contained 1 ml keratin azure suspension and 1 ml appropriately diluted enzyme. The reactions were carried out at 50 °C in a water bath with constant agitation of 200 r/min for 30 min. After incubation, the reactions were stopped by adding 2 ml 0.4 mol/L trichloroacetic acid (TCA) and followed by centrifuging at 3000× g for 20 min to remove the substrate. The supernatant was spectrophotometrically measured for release of the azo dye at 595 nm. The 1 ml keratin azure suspension in the same buffer (like that of the sample) was agitated for 30 min at 50 °C, then was added 2 ml 0.4 mol/L TCA and 1 ml enzyme solution as a control.
 At a TEDx event, TEDTalks video and live speakers combine to spark deep discussion and connection in a small group. In the spirit of ideas worth spreading, TEDx is a program of local, self-organized events that bring people together to share a TED-like experience.
At a TEDx event, TEDTalks video and live speakers combine to spark deep discussion and connection in a small group. In the spirit of ideas worth spreading, TEDx is a program of local, self-organized events that bring people together to share a TED-like experience.
One unit (U) keratinase activity was defined as the amount of enzyme causing 0.01 absorbance increase between the sample and control at 595 nm under the conditions given. Purification of keratinase All operations were performed at room temperature.
After centrifugation at 8000× g for 20 min, solid ammonium sulphate was added to the supernatant to achieve 30% saturation, and then centrifuged to remove the pellet. The enzyme was precipitated from the supernatant by addition of solid ammonium sulphate, with gentle stirring until 80% saturation, and then allowed to stand for 12 h followed by centrifugation at 8000× g.
The pellet was dissolved in 20 mmol/L Tris-HCl buffer (pH 8.0) and applied to a Sephadex G-75 column (6.0 cm×60.0 cm), which was processed at a flow rate of 10 ml/h with 20 mmol/L Tris-HCl buffer (pH 8.0) and every 3 ml fraction was collected. Drajver virtualjnogo com porta dlya usb printera. Active fractions were collected, concentrated by polyethylene glycol 2000 (PEG 2000) and applied to a DEAE (diethylaminoethyl)-Sepharose Fast Flow (FF) column (1.9 cm×20.0 cm). Samples were eluted at a flow rate of 6 ml/min with different concentrations of sodium chloride solution in 20 mmol/L Tris-HCl buffer (pH 8.0) and every 6 ml fraction was collected.